How Many Electrons Can the Third Level Hold
When the third level contains 8 electrons the next two go into the fourth level. How many shells can an atom have.
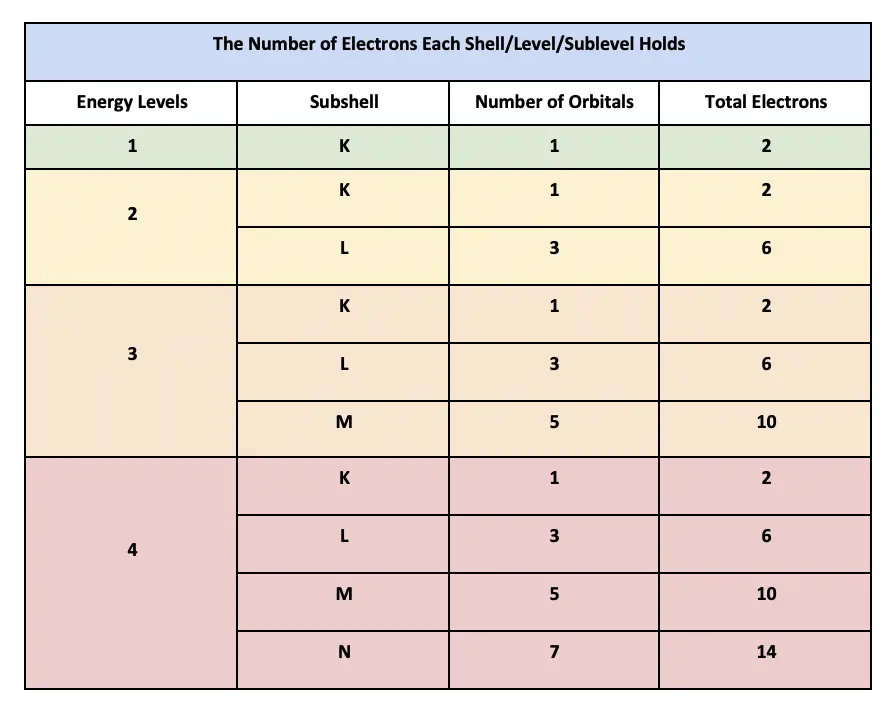
How Many Electrons Are In Each Shell Including 3p Orbitals
It is one of the most important and popular forms of communication.

. Electron shells are a set of feasible states that have the same principal quantum number n the number before the letter on the orbital that the electron can occupy. How many electrons can fit in the third energy level. Electrons are not shared equally in a molecule with unlike atoms.
The third energy level can hold up to 18 electrons meaning that it is not full when it has only electrons. An atom with an nth electron shell can hold 2n2 electrons which is the first shell that can hold 2 electrons the second shell can hold 8 electrons and so on. TV programs provide news information and entertainment to people all over the world.
The tendency of any atom to pull electrons towards itself and away from other atoms is characterized by a quantity called electronegativity. Television or TV is a system for sending moving pictures and sound from one place to another. Before you can understand dipolar interactions you have to know about electronegativity.
How Many Electrons Can The Third Energy Level Hold At Level

Comments
Post a Comment